RoseMarie Gallagher and Paul Ingram Solutions for Chapter: Some Non-Metals and their Compounds, Exercise 2: Q
RoseMarie Gallagher Chemistry Solutions for Exercise - RoseMarie Gallagher and Paul Ingram Solutions for Chapter: Some Non-Metals and their Compounds, Exercise 2: Q
Attempt the free practice questions on Chapter 16: Some Non-Metals and their Compounds, Exercise 2: Q with hints and solutions to strengthen your understanding. Complete Chemistry for Cambridge IGCSE® Second Edition solutions are prepared by Experienced Embibe Experts.
Questions from RoseMarie Gallagher and Paul Ingram Solutions for Chapter: Some Non-Metals and their Compounds, Exercise 2: Q with Hints & Solutions
Ammonia is made from nitrogen and hydrogen. What is the process for making ammonia called?
Ammonia is made from nitrogen and hydrogen. Write an equation for the reaction.
Look at the catalyst beds in the diagram given below. What is in them?
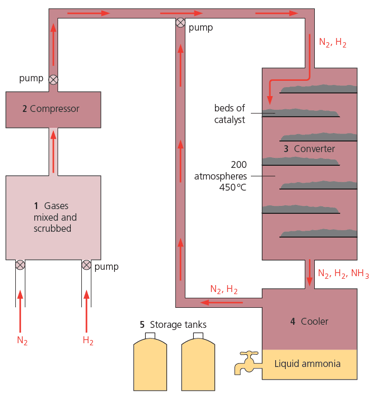
Look at the catalyst beds in the diagram given below. Why are they arranged this way?
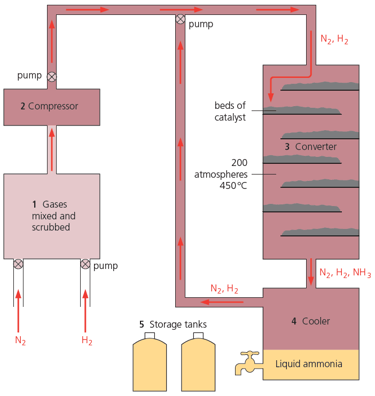
Explain why high pressure and low temperature help the yield, in making ammonia.
400 atmospheres and 250 °C would give a high yield. Why are these conditions not used in the Haber process?
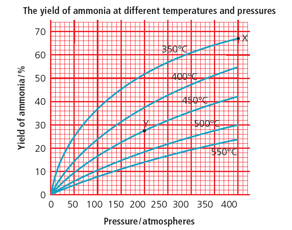
What is the % yield of ammonia at 200 atmospheres and 450 °C? (Use the graph.)
What happens to the unreacted gases in Haber process ?
