Bonding in Carbon-the Covalent Bond
Important Questions on Bonding in Carbon-the Covalent Bond
The picture shows the bonds between atoms in two allotropes of carbon.
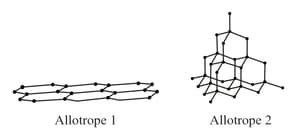
Which allotrope is harder? Explain your answer.
The picture shows the incomplete chain structure of a carbon compound. The second carbon atom has two free electrons.
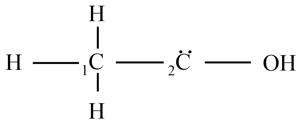
How many oxygen atoms can combine with the second carbon atom to complete the structure?
Which of these molecules contains a double bond?
Carbon compounds have lower boiling points than ionic compounds.
Carbon compounds exist in either saturated or unsaturated form.
Carbon compounds are good conductors of electricity.
Harmera was shown the below images by her chemistry teacher and explained that the blue sphere at the centre is carbon that is bonded with four red spheres that represent hydrogen atoms.
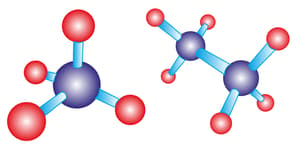
Harmera took a close look and remarked that one carbon has bonded with four hydrogen atoms in the first one and two carbon atoms have been bound with six hydrogen atoms in the second figure. He queried why is it so, that carbon is bound with hydrogen atoms only and not with four different atoms. The teacher then explained him about the valency of various elements which is known as the combining capacity of an element.
From the given figure what could you suggest Harmera about the valency of carbon and hydrogen? What type of bonding is shown in the figure? Explain how this bonding is formed.
Two elements X and Y form a compound. The element X is a main constituent of coal. The amount of this element in the Earth's crust and atmosphere is very small. All living things, plants and animals alike, are composed of this element-based compounds known as organic compounds. As a result, this element is found in all living things.
The element Y is the lightest element. It is colourless, odourless, tasteless, non-toxic, and highly combustible and is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter. Sun, a typical star and the prime source of energy on earth is made up of 74% of this element.
When these two elements react, a compound Z is formed which is a colourless, odourless gas, and is the main constituent of natural gas. It is also a greenhouse gas (GHG), so its presence in the atmosphere affects the earth's temperature and climate system.
Identify , and . Also, discuss the formation of .
The structure shown below has several uses. Which one of the following is a correct use of the given structure:
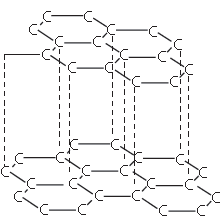
Complete the statement by filling the gaps using appropriate term from the terms given in the bracket.
(slow, coloured, arrow, fast, smell, milky, physical, product, chemical, reactant, covalent, ionic, octet, duplet, exchange, sharing, equality sign)
Electron _____ is complete in each hydrogen in a hydrogen molecule.
A section of the periodic table is shown below. (Except and , the other letters used are not the usual symbols of elements).
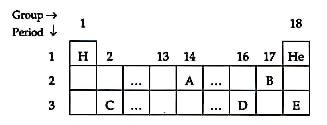
What type of bond do you expect to be formed when A and B combine? (Covalent / Ionic / Coordinate)
An element E exists in three allotropic forms A, B and C. In allotrope A, the atoms of element E are joined to form spherical molecules. In allotrope B, each atom of element E is surrounded by three other E atoms to form a sheet like structure. In allotrope C, each atom of element E is surrounded by four other E atoms to form a rigid structure.
(a) Name the element E.
(b) What is allotrope A?
(c) What is allotrope B?
(d) What is allotrope C?
(e) Which allotrope is used in making jewellery?
(f) Which allotrope is used in making anode of a dry cell?
A solid element X has four electrons in the outermost shell of its atom. An allotrope Y of this element is used as a dry lubricant in machinery and also in making pencil leads.
(a) What is element X?
(b) Name the allotrope Y.
(c) State whether allotrope Y is a good conductor or non-conductor of electricity.
(d) Name one use of allotrope Y (other than lubrication and pencil leads)
(e) Name two other allotropes of element X.
Which of the following is not an ionic-compound?
The atomic numbers of four elements P, Q, R and S are and respectively. Which two elements can combine to form a covalent compound?
A saturated hydrocarbon has fifty hydrogen atoms in its molecule. The number of carbon atoms in its molecule will be:
Explain why, graphite can be used as a lubricant but diamond cannot.

