Exercise
Embibe Experts Science Solutions for Exercise
Simple step-by-step solutions to Exercise questions of Heat from Science Crash Course (Based on Revised Syllabus-2023). Also get 3D topic explainers, cheat sheets, and unlimited doubts solving on EMBIBE.
Questions from Exercise with Hints & Solutions
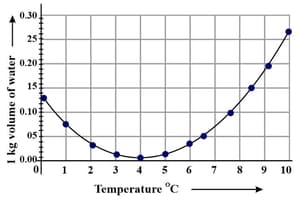
Observe the following graph. Considering the change in volume of water as its temperature is raised from , discuss the difference in the behaviour of water and other substances. What is this behaviour of water called?
Read the following paragraph and answer the question.
If heat is exchanged between a hot and cold object, the temperature of the cold object goes on increasing due to gain of energy and the temperature of the hot object goes on decreasing due to loss of energy. The change in temperature continues till the temperatures of both the objects attain the same value. In this process, the cold object gains heat energy and the hot object loses heat energy. If the system of both the objects is isolated from the environment by keeping it inside a heat resistant box (meaning that the energy exchange takes place between the two objects only), then no energy can flow from inside the box or come into the box.
Which property of the substance is measured using this principle?
When a substance having mass receives of heat, its temperature increases by . What is the specific heat of the substance?
Equal heat is given to two objects and of mass . Temperature of increases by and by . Select the correct statement related to the given situation.
Two substances A and B have specific heats and respectively. If A and B are given and amounts of heat respectively, the change in their temperatures is the same. If the mass of A is , what is the mass of B?
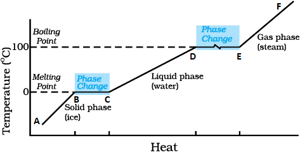
Identify the part representing latent heat of fusion in the temperature vs heat graph for melting of ice.
When of ice at is mixed with of water at in a container, the resulting temperature is . Calculate the latent heat of fusion of ice.
The high specific heat capacity of water is the reason why the ice floats on water.