True Solution, Colloid, Suspension
Important Questions on True Solution, Colloid, Suspension
Most of the juices that we drink are liquids in the colloidal form. Such juices and liquids are available in the market. They do not settle down even after prolonged storage. Some chemical substances are used for this purpose. What are the other purposes for which chemical substances are added in soft drinks?
Most of the juices that we drink are liquids in the colloidal form. Such juices and liquids are available in the market. They do not settle down even after prolonged storage. Some chemical substances are used for this purpose. Are chemicals added to soft drinks only for this purpose?
Most of the juices that we drink are liquids in the colloidal form. Such juices and liquids are available in the market. They do not settle down even after prolonged storage. Do you know that some substances are added for this purpose?
Most of the juices that we drink are liquids in the colloidal form. Such juices and liquids are available in the market. They do not settle down even after prolonged storage. How are they retained as such for long periods of time without settling down?
Tabulate the mixtures that we use in our daily life into solutions, colloids and suspensions. Are colloids and suspensions homogeneous mixtures?
Have you noticed that the path of the light beam can be clearly seen due to the dust particles in a cinema theatre and in smart classrooms where visuals are shown using a projector? What is the reason behind this?
Evaluate the following mixtures: Ink, muddy water, fog, atmospheric air, milk, sugar solution, dilute rice water. Classify them into true solution, colloid and suspension.
Beaker - contains a true solution. Beaker - contains a colloid and Beaker - contains a suspension. Which mixture in these beakers has the biggest particle size?
Beaker - contains a true solution. Beaker- contains a colloid and Beaker - contains a suspension. Which mixture in these beakers has the smallest particle size? How was this identified?
Beaker contains a true solution, beaker contains a colloid and Beaker contains a suspension. Tabulate the characteristics of each mixture in the following table.
| Activity | Solution | Colloid | Suspension |
| Filtering using a filter paper | Particles can not be separated by filtration | _____ | _____ |
| Passing an intense beam of light | The path of the light beam is not visible | The path of the light beam is visible | _____ |
| Keep it undisturbed | _____ | _____ | Particles settle down |
Given below are some activities and observations done by a student using samples of dilute rice water, saltwater, and muddy water.
| Activity | Observation | ||
| Muddy water | Salt water | Dilute rice water | |
| Passing a beam of light | _____ | Path of the beam of light is not visible | _____ |
| Filtering using a filter paper | Components can be separated by filtration | _____ | |
| Keeping undisturbed for some time | _____ | _____ | Particles do not settle down |
a) Complete the table with the missing observations.
b) Classify these samples into solution, colloid, and suspension.
Let's do an experiment. Take equal amounts of water in three separate beakers. Add copper sulphate crystals in the first, milk in the second and chalk powder in the third beaker.
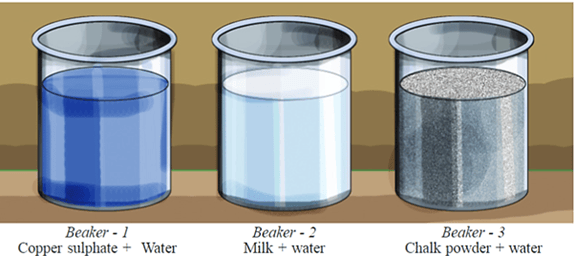
What will you observe if an intense beam of light is passed through the sides of the three beakers? Write your answers in Yes/No in the following table.
| Observation | Beaker- | Beaker- | Beaker- |
| The path of the beam can be observed | |||
| Particles can be observed |
Haven’t you noticed the caption “Shake well before use” in certain medicine bottles?
(a) To which class does the substances in them belong? (Colloid, Solution, Suspension)
(b) What is the relevance of such instructions on these bottles?
Let's do an experiment. Take equal amounts of water in three separate beakers. Add copper sulphate crystals in the first, milk in the second and chalk powder in the third beaker. Stir them well. Keep the beakers undisturbed for some time.
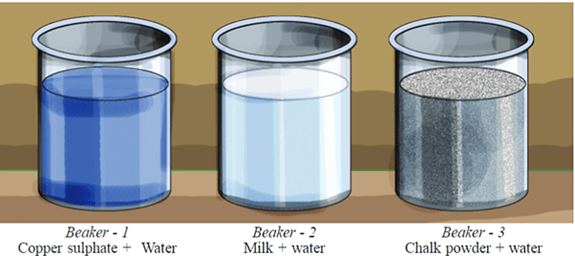
In which of these beakers did the substance settle down?
Classify and tabulate the mixtures given below into solution, colloid and suspension:
Milk, fog, atmospheric air, dilute acid, lime water, ink, smoke.

