Seema Saini Solutions for Chapter: General Principles and Processes of Isolation of Elements, Exercise 1: DPP
Seema Saini Chemistry Solutions for Exercise - Seema Saini Solutions for Chapter: General Principles and Processes of Isolation of Elements, Exercise 1: DPP
Attempt the practice questions on Chapter 1: General Principles and Processes of Isolation of Elements, Exercise 1: DPP with hints and solutions to strengthen your understanding. Chapterwise/Topicwise Daily Practice Problems (DPP) Inorganic Chemistry Part - 2 JEE Main & Advanced solutions are prepared by Experienced Embibe Experts.
Questions from Seema Saini Solutions for Chapter: General Principles and Processes of Isolation of Elements, Exercise 1: DPP with Hints & Solutions
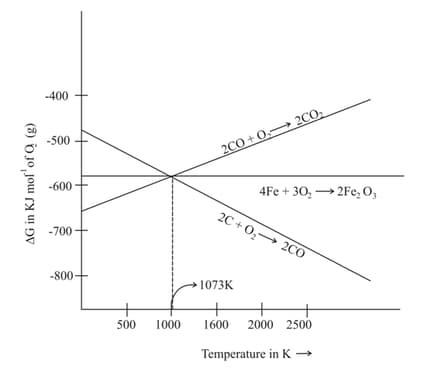
Which of the following is correct regarding the given diagram of reduction of haematite?
vs plot in the Ellingham diagram slopes downward for the reaction:
Consider the following reactions at
Choose the correct statement at .
In many reactions, hydrogen acts as a reducing agent but it is not widely used as a reducing agent for metal extraction. This is because:
At the temperature corresponding to which of the points in the given figure will be reduced to by coupling the reaction with all of the following reactions?
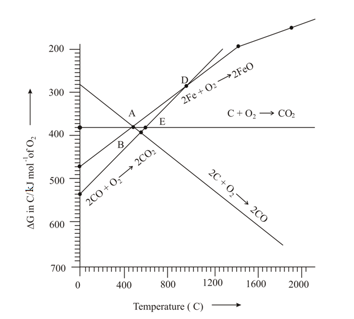
(1)
(2)
(3)
Which of the following statements about the reduction is true?
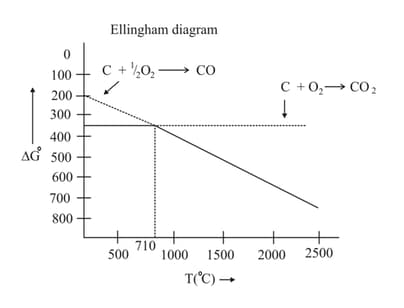
Which of the following is correct on the basis of the above Ellingham diagram for carbon?
Select the incorrect statements about Ellingham diagram.
