Seema Saini Solutions for Chapter: Chemical Kinetics, Exercise 1: DPP 4.1
Seema Saini Chemistry Solutions for Exercise - Seema Saini Solutions for Chapter: Chemical Kinetics, Exercise 1: DPP 4.1
Attempt the free practice questions on Chapter 4: Chemical Kinetics, Exercise 1: DPP 4.1 with hints and solutions to strengthen your understanding. Chapterwise/Topicwise Daily Practice Problems (DPP) Physical Chemistry Part - 2 JEE Main & Advanced solutions are prepared by Experienced Embibe Experts.
Questions from Seema Saini Solutions for Chapter: Chemical Kinetics, Exercise 1: DPP 4.1 with Hints & Solutions
The instantaneous rate of disappearance of the ion in the following reaction is . Then, what will be the rate of appearance of ?
Reaction:
A graph of volume of hydrogen released vs time for the reaction between Zinc and dilute is given in figure. On the basis of this mark the correct statement from the following.
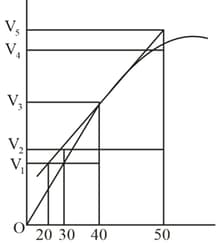
Consider the graph given in figure. Which of the following statements does not show instantaneous rate of reaction at ?

For the reaction , the differential form of the rate law is:
In acidic medium, the rate of reaction between and is given by the expression . What does it mean?
The rate of gaseous reaction is generally expressed in terms of . If it were expressed in terms of change in number of moles per unit time or in terms of change in molar concentration per unit time . Then, which of the following relationship will not hold good?
In the following reaction, which of the following are not the correct expressions regarding how the rate of appearance of the product is related to the disappearance of the reactant?
Which of the following expression(s) cannot be used to describe the instantaneous rate of the reaction ?
