Seema Saini Solutions for Chapter: Chemical Kinetics, Exercise 6: DPP 4.6
Seema Saini Chemistry Solutions for Exercise - Seema Saini Solutions for Chapter: Chemical Kinetics, Exercise 6: DPP 4.6
Attempt the free practice questions on Chapter 4: Chemical Kinetics, Exercise 6: DPP 4.6 with hints and solutions to strengthen your understanding. Chapterwise/Topicwise Daily Practice Problems (DPP) Physical Chemistry Part - 2 JEE Main & Advanced solutions are prepared by Experienced Embibe Experts.
Questions from Seema Saini Solutions for Chapter: Chemical Kinetics, Exercise 6: DPP 4.6 with Hints & Solutions
Acid hydrolysis of the ester is the first-order reaction, and rate constant is given by
,
where and are the volume of standard needed to neutralize acid present at a given time. If ester is neutralised then
Inversion of sucrose is the first-order reaction and is studied by measuring the angle of rotation at different intervals of time
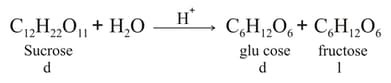 .
.
If and (where, and are the angles of rotation at the start, at the time and at the end of the reaction, respectively), then there is inversion when:
Saponification of ethyl acetate by is studied by titration of the reaction mixture having molar ratio of the reactants. If of is required by of the solution at the start and of is required by another after minutes, then the rate constant is-
Consider the decay of to and by two parallel first-order reactions as shown in the figure
| Reaction | Rate constant | Energy of activation | |
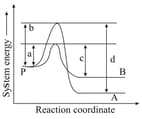
Which of the following is (are) true?
Which of the following statements are correct?
In the reversible reaction, the rate of disappearance of is equal to-
The rate expression for the reaction,
can be derived from the mechanism,
Which of the following statements are correct about rate expression?
The rate of formation of for the forward reaction, is first-order with respect to and each. Which among the following are correct?
