Seema Saini Solutions for Chapter: Solid State, Exercise 2: DPP 1.2
Seema Saini Chemistry Solutions for Exercise - Seema Saini Solutions for Chapter: Solid State, Exercise 2: DPP 1.2
Attempt the free practice questions on Chapter 1: Solid State, Exercise 2: DPP 1.2 with hints and solutions to strengthen your understanding. Chapterwise/Topicwise Daily Practice Problems (DPP) Physical Chemistry Part - 2 JEE Main & Advanced solutions are prepared by Experienced Embibe Experts.
Questions from Seema Saini Solutions for Chapter: Solid State, Exercise 2: DPP 1.2 with Hints & Solutions
Given is the arrangement of atoms in a crystallographic plane. Which plane correctly represent(s) the drawn structure?
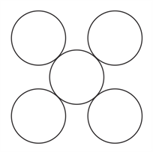
If potassium crystallizes in a body-centered cubic lattice , then
Which of the following is/are correct for the unit cell of element?
Which statements are correct?
If all the atoms are removed from a single axis passing through the center of the cube then choose the incorrect option. If are at the corner of cube and atoms are at face-centered, then
In crystalline solids, atoms or molecules are arranged in regular and long range order fashion in three dimensional pattern. These have sharp melting point, flat faces, sharp edges, bounded by well defined planes. A large number of unit cells, each of which possess a definite geometry bounded by plane faces give rise to the formation of crystal. A point at the corner of unit cell contributes for of each such point of unit cell. A point along the edge contributes for of each such point to unit cell. A body centred point contributes for 1 of such point of unit cell. Coordination number is the number of neighbours that each ion is surrounded by an oppositely charged ions. Radius of the unit cell in sc, fcc and bcc is where is the edge length of the unit cell.
An alloy of and gold crystallizes in cubic lattice in which the gold atoms occupy the lattice points at the corners of cube and copper atoms occupy the center of each face. The formula of this compound is
In crystalline solids, atoms or molecules are arranged in regular and long range order fashion in three dimensional pattern. These have sharp melting point, flat faces, sharp edges, bounded by well defined planes. A large number of unit cells, each of which possess a definite geometry bounded by plane faces give rise to the formation of crystal. A point at the corner of unit cell contributes for of each such point of unit cell. A point along the edge contributes for of each such point to unit cell. A body centred point contributes for 1 of such point of unit cell. Coordination number is the number of neighbours that each ion is surrounded by an oppositely charged ions. Radius of the unit cell in sc, fcc and bcc is where is the edge length of the unit cell.
Packing fraction for a body-centered cubic and simple cubic structure areIn crystalline solids, atoms or molecules are arranged in regular and long range order fashion in three dimensional pattern. These have sharp melting point, flat faces, sharp edges, bounded by well defined planes. A large number of unit cells, each of which possess a definite geometry bounded by plane faces give rise to the formation of crystal. A point at the corner of unit cell contributes for of each such point of unit cell. A point along the edge contributes for of each such point to unit cell. A body centred point contributes for 1 of such point of unit cell. Coordination number is the number of neighbours that each ion is surrounded by an oppositely charged ions. Radius of the unit cell in sc, fcc and bcc is where is the edge length of the unit cell.
An alloy of and gold crystallizes in cubic lattice in which the gold atoms occupy the lattice points at the corners of cube and copper atoms occupy the center of each face. The formula of this compound is
In solid , each molecule has six other molecules as nearest neighbours. of sublimation of at the melting point is and the estimated of sublimation in the absence of -bonding is . The strength of -bond in ion in is:
