Seema Saini Solutions for Chapter: Solutions, Exercise 1: DPP 2.1
Seema Saini Chemistry Solutions for Exercise - Seema Saini Solutions for Chapter: Solutions, Exercise 1: DPP 2.1
Attempt the free practice questions on Chapter 2: Solutions, Exercise 1: DPP 2.1 with hints and solutions to strengthen your understanding. Chapterwise/Topicwise Daily Practice Problems (DPP) Physical Chemistry Part - 2 JEE Main & Advanced solutions are prepared by Experienced Embibe Experts.
Questions from Seema Saini Solutions for Chapter: Solutions, Exercise 1: DPP 2.1 with Hints & Solutions
The normality of phosphorous acid is
What mass of ethanol be added to of water to have the mole fraction of ethanol equal to ?
Molar solubility of helium, nitrogen and oxygen are plotted against partial pressure of the gas at constant temperature. Henry's law constant for these gases will lie in which of the following sequences?
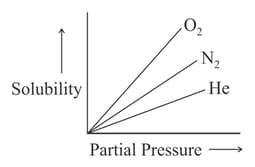
According to William Henry, the solubility of a gas in liquid depends on the pressure of the gas. If '' is the molality of the gas and '' is its pressure, then which of the following plot is in accordance with the law?
Which of the following plots is correct for solutions of different solutes having the same density?
is molarity and is molar mass of the solute.
The molarity of a solution obtained by mixing of with of will be:
The vapour pressure of a dilute solution of a solute is influenced by
By adding water to the solution of an ionic compound, its
