Seema Saini Solutions for Chapter: Solutions, Exercise 4: DPP 2.4
Seema Saini Chemistry Solutions for Exercise - Seema Saini Solutions for Chapter: Solutions, Exercise 4: DPP 2.4
Attempt the free practice questions on Chapter 2: Solutions, Exercise 4: DPP 2.4 with hints and solutions to strengthen your understanding. Chapterwise/Topicwise Daily Practice Problems (DPP) Physical Chemistry Part - 2 JEE Main & Advanced solutions are prepared by Experienced Embibe Experts.
Questions from Seema Saini Solutions for Chapter: Solutions, Exercise 4: DPP 2.4 with Hints & Solutions
In which case, depression freezing point is equal to cryoscopic constant for water?
The amount of urea to be dissolved in of water to produce a depression of in the freezing point is:
Freezing point of an aqueous solution is . Elevation of boiling point of same solution would be
The amount of ice that will separate on cooling a solution containing of ethylene glycol in water to is: is
What should be the freezing point of aqueous solution containing of in of water ?
The phase diagrams for a pure solvent (represented by the solid line) and a corresponding solution (containing a non-volatile solute and represented by the dashed lines) are shown below.
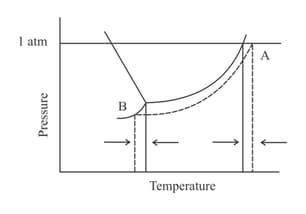
Choose the correct option.
Where and stand for freezing point temperature, boiling point temperature and molality.
A solution of urea (mol mass ) boils at at atmospheric pressure. If and for water are and respectively, the above solution will not freeze at:
Which of the following is/are not linked to the lowering of vapor pressure by a non-volatile solute?
