Seema Saini Solutions for Chapter: Solutions, Exercise 5: DPP 2.5
Seema Saini Chemistry Solutions for Exercise - Seema Saini Solutions for Chapter: Solutions, Exercise 5: DPP 2.5
Attempt the free practice questions on Chapter 2: Solutions, Exercise 5: DPP 2.5 with hints and solutions to strengthen your understanding. Chapterwise/Topicwise Daily Practice Problems (DPP) Physical Chemistry Part - 2 JEE Main & Advanced solutions are prepared by Experienced Embibe Experts.
Questions from Seema Saini Solutions for Chapter: Solutions, Exercise 5: DPP 2.5 with Hints & Solutions
Insulin is dissolved in a suitable solvent and the osmotic pressure () of solutions of various concentrations is measured at . The slope of a plot of against is found to be . The molar mass of the insulin is
What is the osmolarity of a solution?
Study the following figure and choose the correct option.
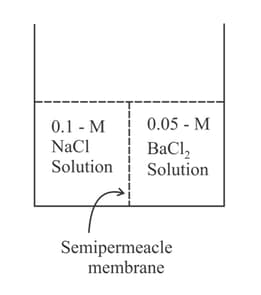
Study the figure given below and pick out the correct options of the following.
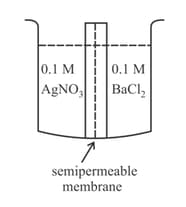
In which of the following solutions, permits the solvent molecules to pass through?
Which statement(s) is/are correct about osmotic pressure (), volume () and temperature ()?
The slope of vs was made against insulin concentration in and temperature . If is in and the slope of line obtained is , which of the following are not the molar mass (in ) of insulin?
A graph showing the variation of osmotic pressure versus molar concentration of an aqueous solution at temperature is given below.
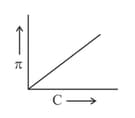
The slope of the line doesn't represent
