Seema Saini Solutions for Chapter: Atomic Structure, Exercise 2: DPP 3.2
Seema Saini Chemistry Solutions for Exercise - Seema Saini Solutions for Chapter: Atomic Structure, Exercise 2: DPP 3.2
Attempt the free practice questions on Chapter 3: Atomic Structure, Exercise 2: DPP 3.2 with hints and solutions to strengthen your understanding. Chapterwise/Topicwise Daily Practice Problems (DPP) Physical Chemistry Part 1 JEE Main & Advanced solutions are prepared by Experienced Embibe Experts.
Questions from Seema Saini Solutions for Chapter: Atomic Structure, Exercise 2: DPP 3.2 with Hints & Solutions
Suppose that a hypothetical atom gives a red, green, blue and violet line in the spectrum. Which jump according to figure would give off the red spectral line?
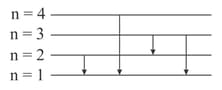
For the following transitions in hydrogen-like atoms, select the correct relation(s):
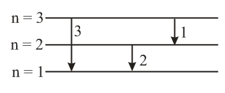
If the electron of the hydrogen atom is replaced by another particle of the same charge but of the double mass, then:
Let and are the radius of the orbit, speed of the electron and the total energy of the electron, respectively. Which of the following quantities are proportional to the quantum number
Select the correct statements for hydrogen like atoms or ions.
An electron in a hydrogen atom makes a transition from to The time period of the electron in the initial state is eight times that in the final state. What are the possible values of and
Identify the correct statement(s) from the following:
A hydrogen-like atom has ground state binding energy . Then:
