Seema Saini Solutions for Chapter: States of Matter, Exercise 4: DPP 4.4
Seema Saini Chemistry Solutions for Exercise - Seema Saini Solutions for Chapter: States of Matter, Exercise 4: DPP 4.4
Attempt the free practice questions on Chapter 4: States of Matter, Exercise 4: DPP 4.4 with hints and solutions to strengthen your understanding. Chapterwise/Topicwise Daily Practice Problems (DPP) Physical Chemistry Part 1 JEE Main & Advanced solutions are prepared by Experienced Embibe Experts.
Questions from Seema Saini Solutions for Chapter: States of Matter, Exercise 4: DPP 4.4 with Hints & Solutions
The relative ratio of at a given temperature is:
The most probable speed of an ideal gaseous molecules at is . The average speed at would be
The root square speed of a gaseous molecule is given as . This shows that at a given temperature
Which of the following expressions correctly represents the relationship between the average kinetic energy of and molecules at the same temperature?
A mixture containing and in a vessel at has a total translation kinetic energy. The total mass of mixture is . What is the mass of in mixture?
Which of the following graphs is in accordance with the Maxwell distribution of molecular velocities and its dependence on temperature?
Fraction of molecules are plotted against velocity of molecules, according to Maxwell-Boltzmann distribution law. The velocity corresponding to the points and are:
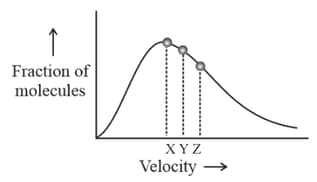
Here,
most probable velocity.
average velocity.
root mean square velocity.
Which of the following facts regarding an ideal gas is/are correct?
