Seema Saini Solutions for Chapter: States of Matter, Exercise 5: DPP 4.5
Seema Saini Chemistry Solutions for Exercise - Seema Saini Solutions for Chapter: States of Matter, Exercise 5: DPP 4.5
Attempt the free practice questions on Chapter 4: States of Matter, Exercise 5: DPP 4.5 with hints and solutions to strengthen your understanding. Chapterwise/Topicwise Daily Practice Problems (DPP) Physical Chemistry Part 1 JEE Main & Advanced solutions are prepared by Experienced Embibe Experts.
Questions from Seema Saini Solutions for Chapter: States of Matter, Exercise 5: DPP 4.5 with Hints & Solutions
The value of compressibility factor of a Van der Waal's gas at critical point is:
The pressure exerted by of at is , assuming that volume occupied by molecules is negligible. The value of Van der Waal's constant for attraction of gas is:
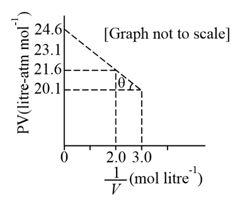
For one mole of a Van der Waal's gas when, and , the vs plot is shown below. The value of the Van der Waal's constant is:
Which of the following statements are correct?
Which of the following statements are not correct?
Which of the following statements are correct?
Which of the following statements is/are correct about real gases?
At a very high pressure, Van der Waal's equation reduces to:
