Seema Saini Solutions for Chapter: Thermodynamics, Exercise 3: DPP 5.3
Seema Saini Chemistry Solutions for Exercise - Seema Saini Solutions for Chapter: Thermodynamics, Exercise 3: DPP 5.3
Attempt the free practice questions on Chapter 5: Thermodynamics, Exercise 3: DPP 5.3 with hints and solutions to strengthen your understanding. Chapterwise/Topicwise Daily Practice Problems (DPP) Physical Chemistry Part 1 JEE Main & Advanced solutions are prepared by Experienced Embibe Experts.
Questions from Seema Saini Solutions for Chapter: Thermodynamics, Exercise 3: DPP 5.3 with Hints & Solutions
The heat of neutralization of a strong acid and a strong alkali is . The heat released when of solution is mixed with of is
One mole of soda ash dissolves in water with the evolution of of heat. Dissolution of of the hydrate crystals in water resulted in the absorption of of heat. The heat released due to hydration of soda ash is:
Which of the following statements is/are correct?
Which of the following statements are correct?
Which of the following mixtures on neutralization will show a same rise in temperature?
Which of the following are incorrect?
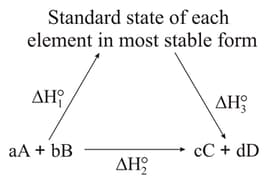
Which of the following reactions are exothermic?
Select the correct statements.
