Seema Saini Solutions for Chapter: Thermodynamics, Exercise 5: DPP 5.5
Seema Saini Chemistry Solutions for Exercise - Seema Saini Solutions for Chapter: Thermodynamics, Exercise 5: DPP 5.5
Attempt the free practice questions on Chapter 5: Thermodynamics, Exercise 5: DPP 5.5 with hints and solutions to strengthen your understanding. Chapterwise/Topicwise Daily Practice Problems (DPP) Physical Chemistry Part 1 JEE Main & Advanced solutions are prepared by Experienced Embibe Experts.
Questions from Seema Saini Solutions for Chapter: Thermodynamics, Exercise 5: DPP 5.5 with Hints & Solutions
Predict which of the following reaction(s) has a positive entropy change?
When two moles of an ideal gas is heated from to at constant pressure, the change in entropy of gas is
For the reaction:
What is the initial free energy change?
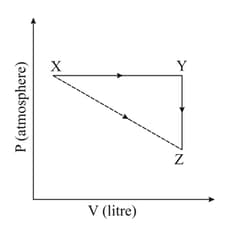
For an ideal gas, consider only work in going from an initial state to the final state . The final state can be reached by either of the two paths shown in the figure. Which of the following choice(s) is(are) correct? [Take as change in entropy and as work done.]
A reaction attains equilibrium state under standard conditions, then:
Select the correct statement for the equilibrium under standard conditions:
In which of the following process does entropy increase?
In which of the following reaction is ?
