Sergey Bylikin, Gary Horner and, Brian Murphy Solutions for Chapter: Redox Processes (AHL), Exercise 6: Questions
Sergey Bylikin Chemistry Solutions for Exercise - Sergey Bylikin, Gary Horner and, Brian Murphy Solutions for Chapter: Redox Processes (AHL), Exercise 6: Questions
Attempt the practice questions on Chapter 19: Redox Processes (AHL), Exercise 6: Questions with hints and solutions to strengthen your understanding. Oxford IB Diploma Programme Chemistry Course Companion solutions are prepared by Experienced Embibe Experts.
Questions from Sergey Bylikin, Gary Horner and, Brian Murphy Solutions for Chapter: Redox Processes (AHL), Exercise 6: Questions with Hints & Solutions
Construct and annotate the electrolytic cell for the electrolysis of concentrated potassium iodide.
Identify the direction of the movement of electrons and ion flow.
Construct and annotate the electrolytic cell for the electrolysis of concentrated potassium iodide.
State what would be observed at each electrode.
A current of is passed through an electrolytic cell for the electrolysis of water, using a dilute sulphuric acid solution, for a duration of .
Construct and annotate a suitable electrolytic cell for this experiment.
A current of is passed through an electrolytic cell for the electrolysis of water, using a dilute sulfuric acid solution, for a duration of .
Identify the half-equations occurring at the cathode and anode electrodes and the equation for the overall cell reaction.
A current of is passed through an electrolytic cell for the electrolysis of water, using a dilute sulphuric acid solution, for a duration of .
Identify the direction of the movement of electrons and ion flow.
A current of is passed through an electrolytic cell for the electrolysis of water, using a dilute sulphuric acid solution, for a duration of .
Determine the volume, in , of the two gases generated in the process at SATP conditions.
Electroplating is an important application of electrolytic cells with commercial implications. Copper may be plated using an electrolytic cell with an aqueous acidified copper(II) sulfate electrolyte. For the copper plating of tin to make jewellery, state the half-equation at each electrode.
Assume the tin electrode is inert. Suggest two observations that you would be able to make as the electroplating progresses.
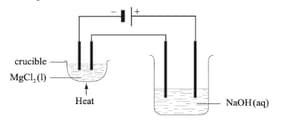
Two electrolytic cells are connected in series as shown in the diagram below. In one there is molten magnesium chloride and in the other, dilute sodium hydroxide solution. Both cells have inert electrodes. If of magnesium is produced in the first cell, deduce the identity and mass of products produced at the positive and negative electrodes in the second cell.