Sergey Bylikin, Gary Horner and, Brian Murphy Solutions for Chapter: Redox Processes, Exercise 13: Questions
Sergey Bylikin Chemistry Solutions for Exercise - Sergey Bylikin, Gary Horner and, Brian Murphy Solutions for Chapter: Redox Processes, Exercise 13: Questions
Attempt the practice questions on Chapter 9: Redox Processes, Exercise 13: Questions with hints and solutions to strengthen your understanding. Oxford IB Diploma Programme Chemistry Course Companion solutions are prepared by Experienced Embibe Experts.
Questions from Sergey Bylikin, Gary Horner and, Brian Murphy Solutions for Chapter: Redox Processes, Exercise 13: Questions with Hints & Solutions
Which statement about the electrolysis of molten sodium chloride is correct?
What is the reducing agent in the reaction below?
.
Which changes could take place at the positive electrode (cathode) in a voltaic cell?
I. to .
II. to .
III. to .
Metal A is more reactive than metal B. A standard voltaic cell is made as shown figure.
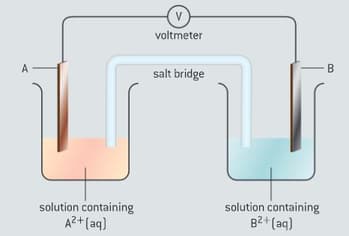
Which statement is correct?
A sample of an alkali metal iodate, , was dissolved in water, acidified, and an excess of potassium iodide, added. The resulting iodine solution required of the sodium thiosulphate pentahydrate solution, for complete titration using starch solution as an indicator. Calculate the relative atomic mass of and hence identify the metal. Deduce all relevant half-equations involved.
Describe how the dissolved oxygen concentration in a river would decrease if a car factory releases warm water into the river after using it for cooling.
Describe how the dissolved oxygen concentration in a river would decrease if a farmer puts large quantities of a fertilizer on a field next to the river.
Describe how the addition of nitrates or phosphates to water can increase the BOD value of a water sample.
