Shikha Goel and Saleha Parvez Solutions for Chapter: The Periodic Table, Exercise 7: CHALLENGERS*
Shikha Goel Chemistry Solutions for Exercise - Shikha Goel and Saleha Parvez Solutions for Chapter: The Periodic Table, Exercise 7: CHALLENGERS*
Attempt the practice questions on Chapter 5: The Periodic Table, Exercise 7: CHALLENGERS* with hints and solutions to strengthen your understanding. All In One ICSE Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Shikha Goel and Saleha Parvez Solutions for Chapter: The Periodic Table, Exercise 7: CHALLENGERS* with Hints & Solutions
Consider the following statements.
I. Dobereiner's triads had a great significance in predicting atomic mass and properties of middle element.
II. Law of octaves is based upon octaves found in music.
III. Eka-boron and eka-aluminium were named as scandium and gallium, later on.
Identify the correct statements.
are three members of a Dobereiner's triad If the atomic mass of is and that of is . What is the atomic mass of ?.
Mendeleev's periodic table is based upon the atomic masses. This periodic table is upset by the fact that:
In terms of period and group where would you locate the element with ?
According to IUPAC nomenclature, a newly discovered element has been named as . This atomic number of the element is
A trend common to both of group I and VII element in the periodic table as atomic number increases is
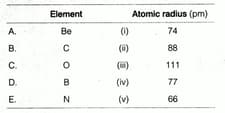
Match the correct atomic radius with the element.
Consider the following statements regarding periodic classification of elements.
I. The properties of elements are periodic functions of their atomic numbers.
II. Non-metallic elements are less in number than metallic elements.
Which is correct statement(s)?
