Olympiad/HOTS corner
Subject Experts Chemistry Solutions for Exercise - Olympiad/HOTS corner
Simple step-by-step solutions to Olympiad/HOTS corner questions of Metals And Non-metals from Foundation Course Chemistry. Also get 3D topic explainers, cheat sheets, and unlimited doubts solving on EMBIBE.
Questions from Olympiad/HOTS corner with Hints & Solutions
Mention the composition and uses of the solder alloy.
Which gas is produced when an acid reacts with a metal?
A few chemical properties of three metals X, Y and Z are summarised in the given table.
| Metal | Chemical Properties |
| X | Reacts with hot water and starts floating on the surface of water. |
| Y | Reacts with steam. |
| Z | Does not react with dilute acids as well as steam. |
The methods which can be used to extract X, Y and Z from their ores are respectively,
Mitaali, a class student wanted to study the speed of reactions of different acids with zinc metal. For this, she conducted the following experiments using zinc metal in excess.
| Experiment | Acid Used |
| I. | of . |
| II. | of . |
| III. | of . |
She measured the volume of gas produced at regular time intervals and summarised her results in a graphical form. Which of the following graphs best represents the results obtained in experiments I, II and III?
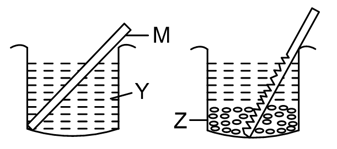
A metal rod M was dipped in a coloured solution Y. After some time it was observed that the metal rod starts dissolving in the solution and the solution starts fading in colour. However, a coloured precipitate Z was seen at the bottom of the beaker. M, Y and Z could be
Name a non-metal which is lustrous.
Main objective of smelting of ore is
A metal is placed below and above , in activity series. The extraction of metal is done by reacting carbon with its oxide. Metal oxide is used to join cracks of machine parts and rail lines by reacting it with . The metal is
