What is a Solution?
Important Questions on What is a Solution?
Avinash and Pratiksha were doing experiments in a chemistry lab. Avinash took of water at room temperature in a beaker and dissolved a little solid S in it by stirring to obtain a solution X. Then Pratiksha started adding more and more solid S to the solution with constant stirring while keeping the temperature of the solution constant at . At the same time, Pratiksha and Avinash observed that no more solid dissolves in water and, at the same time, some solid is also left undissolved at the bottom of the beaker. So, they separated the contents of the beaker by filtering them through filter paper, and they got solution Y in the form of a filtrate.

Please answer the below question by understanding the information given above.
(a) What type of solution is X?
(b) What type of solution is Y?
(c) What will you observe if the solution Y at is cooled down to by keeping the beaker in crushed ice? Give a reason for this observation.
(d) Give the term used to denote "the amount of solids dissolved in a given water".
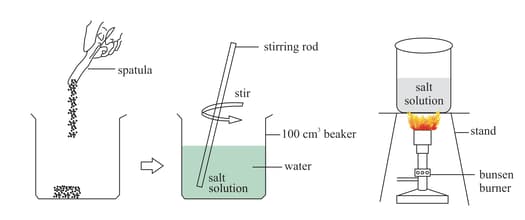
Take three beakers and fill them with water. Add two spoons of salt to each of them. Place the first beaker undisturbed, stir the second and warm the third beaker. What do you observe from the above three situations?
(a) Which method allows the solute to dissolve in the solvent easily?
(b) If you increase the temperature of the third beaker, what will happen?
(c) Repeat the activity by using salt crystals instead of salt powder. What change do you observe
An experiment on the solubility of solids in liquid was carried out by a group of pupils. They took several salts and dissolved them in water separately. These solutions were gradually heated in a separate beaker, and the solubility was measured at various temperatures. For many salts, a graph has been plotted against solubility vs temperature. The graph below depicts the solubility of various salts at various temperatures
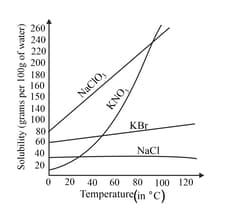
From the information given in the graph conclude the general effect of temperature on the solubility of solids in liquids? Also find the salt that is almost equally soluble at all temperatures?
Ms. Radhika taught her pupils about concentration of solutions and in order to demonstrate this concept she formed two groups. The first group was asked to dissolve of sugar in of water while the second group was asked to dissolve of sugar in water to make of solution. The two groups are now asked to find the mass of the solutions prepared by them. Can you tell the difference in the mass percentage of the two solutions prepared by these groups?.

Lohit, Navika, Naveen, Shilpa, and Abhishek went to an industry visit with their teacher. They went to the chemical industry and visited all the departments in the industry. At last, they went to the testing department, where a group of chemists were testing the newly produced chemicals. They saw that one of the chemists was doing a test by dissolving some chemicals in the water at different temperatures. They asked their teacher about this test, and they requested their teacher to perform this experiment at their school laboratory.

The next day in school, they took three chemicals and performed solubility tests at different temperatures, and created this tabular column.
Table: Variation of solubility with temperature in grams per grams of water.
| Salt | C | C | C | C | C | C |
The teacher then asked them if a solution containing of water and of was heated to , how many additional grams of must be added to prepare a saturated solution at ?

