Umakant Kondapure, Collin Fernandes, Nipun Bhatia, Vikram Bathula and, Ketki Deshpande Solutions for Chapter: Kinetic Theory of Gases and Radiations, Exercise 3: Competitive Thinking
Umakant Kondapure Physics Solutions for Exercise - Umakant Kondapure, Collin Fernandes, Nipun Bhatia, Vikram Bathula and, Ketki Deshpande Solutions for Chapter: Kinetic Theory of Gases and Radiations, Exercise 3: Competitive Thinking
Attempt the practice questions on Chapter 3: Kinetic Theory of Gases and Radiations, Exercise 3: Competitive Thinking with hints and solutions to strengthen your understanding. MHT-CET TRIUMPH Physics Multiple Choice Questions Part - 2 Based on Std. XI & XII Syllabus of MHT-CET solutions are prepared by Experienced Embibe Experts.
Questions from Umakant Kondapure, Collin Fernandes, Nipun Bhatia, Vikram Bathula and, Ketki Deshpande Solutions for Chapter: Kinetic Theory of Gases and Radiations, Exercise 3: Competitive Thinking with Hints & Solutions
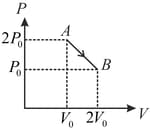
moles of an ideal gas undergoes a process as shown in the figure. The maximum temperature of the gas during the process will be
The amount of heat energy required to raise the temperature of of Helium at N.T.P., from to is
At what temperature will the RMS speed of oxygen molecules become just sufficient for escaping from the Earth's atmosphere? (Given: Mass of oxygen molecule , Boltzmann's constant
Assertion: Thermodynamic processes in nature are irreversible.
Reason: Dissipative effects cannot be eliminated.
Assertion: In an isolated system, the entropy increases.
Reason: The processes in an isolated system are adiabatic.
One mole of an ideal gas requires heat to raise the temperature by when heated at constant pressure. If the same gas is heated at constant volume to raise the temperature by then heat required is [given gas constant .]
Consider a spherical shell of radius at temperature . The black body radiation inside it can be considered as an ideal gas of photons with internal energy per unit volume and pressure . If the shell now undergoes an adiabatic expansion the relation between and is
The power radiated by a black body is $P$ and it radiates maximum energy at wavelength, $\lambda_{0}$. If the temperature of the black body is now changed so that it radiates maximum energy at wavelength $\frac{3}{4} \lambda_{0},$ the power radiated by it becomes . The value of is
