D. C. Pandey Solutions for Chapter: Laws of Thermodynamics, Exercise 5: Exercises
D. C. Pandey Physics Solutions for Exercise - D. C. Pandey Solutions for Chapter: Laws of Thermodynamics, Exercise 5: Exercises
Attempt the practice questions on Chapter 5: Laws of Thermodynamics, Exercise 5: Exercises with hints and solutions to strengthen your understanding. Understanding Physics JEE Main & Advanced WAVES AND THERMODYNAMICS solutions are prepared by Experienced Embibe Experts.
Questions from D. C. Pandey Solutions for Chapter: Laws of Thermodynamics, Exercise 5: Exercises with Hints & Solutions
The temperature of a monatomic gas is increased from to in three different processes such as isochoric, isobaric and adiabatic. Heat given to the gas in these three processes are , and , respectively. Then, choose the correct option.
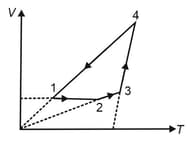
A cyclic process is depicted on diagram. The and diagrams for this cyclic process are given below. Select the correct choices (more than one options is/are correct)
One mole of a monatomic ideal gas is taken along the cycle as shown in the diagram.
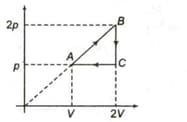
The ratio of specific heat in the process to the specific heat in the process is
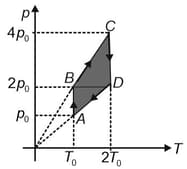
One mole of a monoatomic ideal gas is taken through the cycle as shown in the figure.
The temperature at is
One mole of a monoatomic ideal gas is taken through the cycle as shown in the figure.
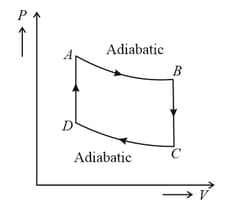
Work done by gas in the process is
One mole of a monoatomic ideal gas is taken through the cycle as shown in the figure.
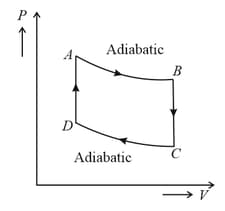
Heat lost by the gas in the process is
The pressure versus temperature graph of of an ideal gas is shown in the figure. Plot the corresponding,
density versus volume graph
pressure versus volume graph and
density versus pressure graph.
Three moles of an ideal gas being initially at a temperature were isothermally expanded times its initial volume and then isochorically heated so that the pressure in the final state becomes equal to that in the initial state. The total heat supplied in the process is . Find of the gas.