V K Sally and R Goel Solutions for Chapter: Structure of Atom, Exercise 2: EXERCISE 4B
V K Sally Chemistry Solutions for Exercise - V K Sally and R Goel Solutions for Chapter: Structure of Atom, Exercise 2: EXERCISE 4B
Attempt the free practice questions on Chapter 4: Structure of Atom, Exercise 2: EXERCISE 4B with hints and solutions to strengthen your understanding. Core science chemistry solutions are prepared by Experienced Embibe Experts.
Questions from V K Sally and R Goel Solutions for Chapter: Structure of Atom, Exercise 2: EXERCISE 4B with Hints & Solutions
, and are the isotopes of hydrogen. Explain:
(i) Why do these isotopes have similar chemical properties?
(ii) Why are these isotopes electrically neutral?
The ratio of isotopes of , and in natural chlorine is 1:3. Calculate the fractional atomic mass of chlorine.
Find out the valency of the atoms represented by the Fig. (i) and (ii).
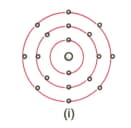
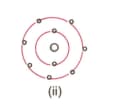
Why do helium, neon and argon have a zero valency?
1. , and are the isotopes of neon.
(i) What do the subscripts and superscripts represent?
(ii) What is the cause of change in superscripts?
(iii) Give the geometric diagram of .
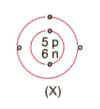
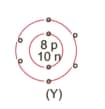
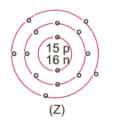
What information do you get from the figure given about the atomic number, mass number and valency of atoms X, Y, and Z?. Give your answer in a tabular form.
An element E is available in the form of two isotopes (75.8%) and (24.2%) Calculate the average atomic mass of element E.
The average atomic mass of a sample of an element X is 23.04 m. What are the percentage of isotopes in the sample?
