MEDIUM
Earn 100
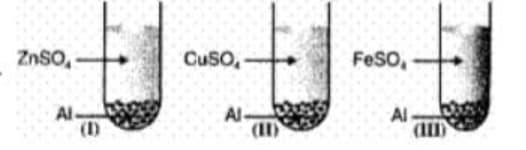
Observation
I
II
III
Solution after reaction
Colourless
Colourless
Colourless
Metal Deposited
Which of the following is correct conclusion?

(a) is more reactive than and but less reactive than
(b) is more reactive than but less reactive than and
(c) is more reactive than and but less reactive than
(d)is more reactive than
50% studentsanswered this correctly
Important Questions on Metals and Non-Metals
EASY
Give reason:
Metals like sodium and potassium are stored under oil.
MEDIUM
HARD
HARD
MEDIUM
HARD
HARD
MEDIUM
MEDIUM
HARD
HARD
Magnesium, Copper, Iron, Sodium, Zinc, Lead, Calcium.
HARD
HARD
HARD
MEDIUM
MEDIUM
HARD
MEDIUM
MEDIUM
MEDIUM
(a) Name the elements which have taken the place of these elements.
(b) Mention the group and the period of these elements in the modern periodic table.
(c) How many valence electrons are present in each one of them?

