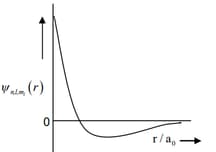
HARD
11th CBSE
IMPORTANT
Earn 100
Column 1
Column 2
Column 3
(I) orbital
(i)
(II) orbital
(ii) One radial node
(Q) Probability density at nucleus
(III) orbital
(iii)
(R) Probability density is maximum at nucleus
(IV) orbital
(iv) -plane is a nodal plane
(S) Energy needed to excite electron from
state to state is times the energy needed to excite electron from state to state
For ion, the only INCORRECT combination is

state to state is times the energy needed to excite electron from state to state
(a)
(b)
(c)
(d)
60% studentsanswered this correctly

Important Questions on Structure of Atom
HARD
11th CBSE
IMPORTANT
| Column 1 | Column 2 | Column 3 |
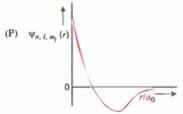
| (I) orbital | (i) | 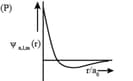 |
| (II) orbital | (ii) One radial node | (Q) Probability density at nucleus |
| (III) orbital | (iii) | (R) Probability density is maximum at nucleus |
| (IV) orbital | (iv) -plane is a nodal plane | (S) Energy needed to excite electron from state to state is times the energy needed to excite electron from state to state |
For the given orbital in column, the only CORRECT combination for any hydrogen-like species is
HARD
11th CBSE
IMPORTANT
| Column 1 | Column 2 | Column 3 |
| (I) orbital | (i) |
(P)
|
| (II) orbital | (ii) One radial node | (Q) Probability density at nucleus |
| (III) orbital | (iii) | (R) Probability density is maximum at nucleus |
| (IV) orbital | (iv) -plane is a nodal plane |
(S) Energy needed to excite electron from |
For the hydrogen atom, the only CORRECT combination is
MEDIUM
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
The atomic spectrum of hydrogen is found to contain a series of lines of wavelengths. The wavelength (in nm) of the next line in the series will be _____.