of urea and of glucose are dissolved of water find the boiling point of solutions and pure water boiling point is . for water is .
Important Questions on Solutions
The plot given below shows curves (where, is the pressure and is the temperature) for two solvents and and isomolal solutions of in these solvents. completely dissociates in both the solvents.
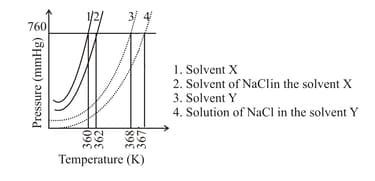
On the addition of equal number of moles of a non-volatile solute in equal amount (in ) of these solvents, the elevation of boiling point of solvent is three times that of solvent . The solute is known to undergo dimerization in these solvents. If the degree of dimerization is in the solvent , the degree of dimerization in the solvent is _______.
for water is
[Assume ionisation of the complex and coordination number of as and that all molecules are present inside the coordination sphere]
is dissociated in water to and . The boiling point of molal aqueous solution of is-- . (Round off to the Nearest Integer).
[Given : Molal elevation constant of water boiling point of pure water]
A solute dimerizes in water. The boiling point of a molal solution of is . The percentage association of is ___ .
(Round off to the Nearest integer)
Use : for water
Boiling point of water

