Adiabatic expansions of an ideal gas is accompanied by
Adiabatic expansions of an ideal gas is accompanied by
decrease in
Increase in temperature
decrease in
no change in any one of the above properties
Important Questions on Equilibrium
For this equilibrium, the correct statement(s) is/are
If we show a red litmus paper over ammonia gas, it turns blue.
Which property of ammonia is shown here?
The rate of this reaction is increased by
At equilibrium, the mass of each of the reactants and products remains constant.
At equilibrium, the rate of forward reaction is equal to the rate of backward reaction.
Using the data provided, find the value of equilibrium constant for the following reaction at and atm pressure.
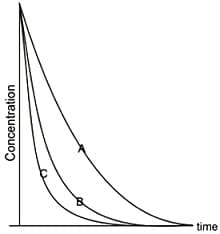
These profiles imply that the decay constants and follow the order
A reaction between carbon and oxygen is represented by . In which of the type(s), the above reaction can be classified?
i) Combination reaction
ii) Combustion reaction
iii) Decomposition reaction
iv) Irreversible reaction
The equilibrium constant for the reaction, is . If the rate constant for the forward reaction is , the rate constant for the backward reaction is :

