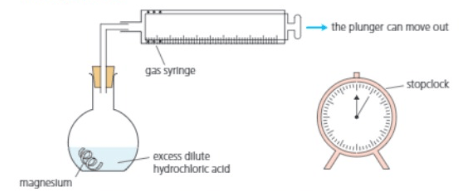
Clean the magnesium with sandpaper. Put dilute hydrochloric acid in the flask. Drop the magnesium into the flask, and insert the stopper and syringe immediately. Start the clock at the same time.
Hydrogen begins to bubble off. It rises up the flask and into the gas syringe, pushing the plunger out:

At the start, no gas has yet been produced or collected. So the plunger is all the way in.

Now the plunger has been pushed out to the mark. of gas have been collected.
For the above experiment. Explain why the clock is started the moment the reactants are mixed.




Important Questions on The Speed of a Reaction
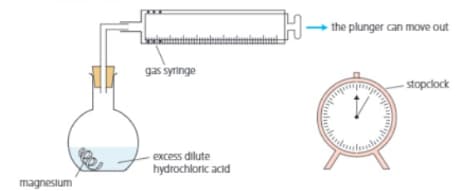
Clean the magnesium with sandpaper. Put dilute hydrochloric acid in the flask. Drop the magnesium into the flask, and insert the stopper and syringe immediately. Start the clock at the same time.
Hydrogen begins to bubble off. It rises up the flask and into the gas syringe, pushing the plunger out:

At the start, no gas has yet been produced or collected. So the plunger is all the way in.

Now the plunger has been pushed out to the mark. of gas have been collected.
For the above experiment. Explain why the stopper is replaced immediately.
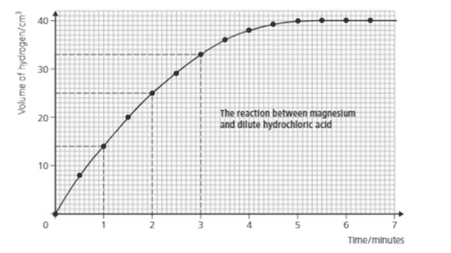
From the above graph how can you tell when the reaction is over?
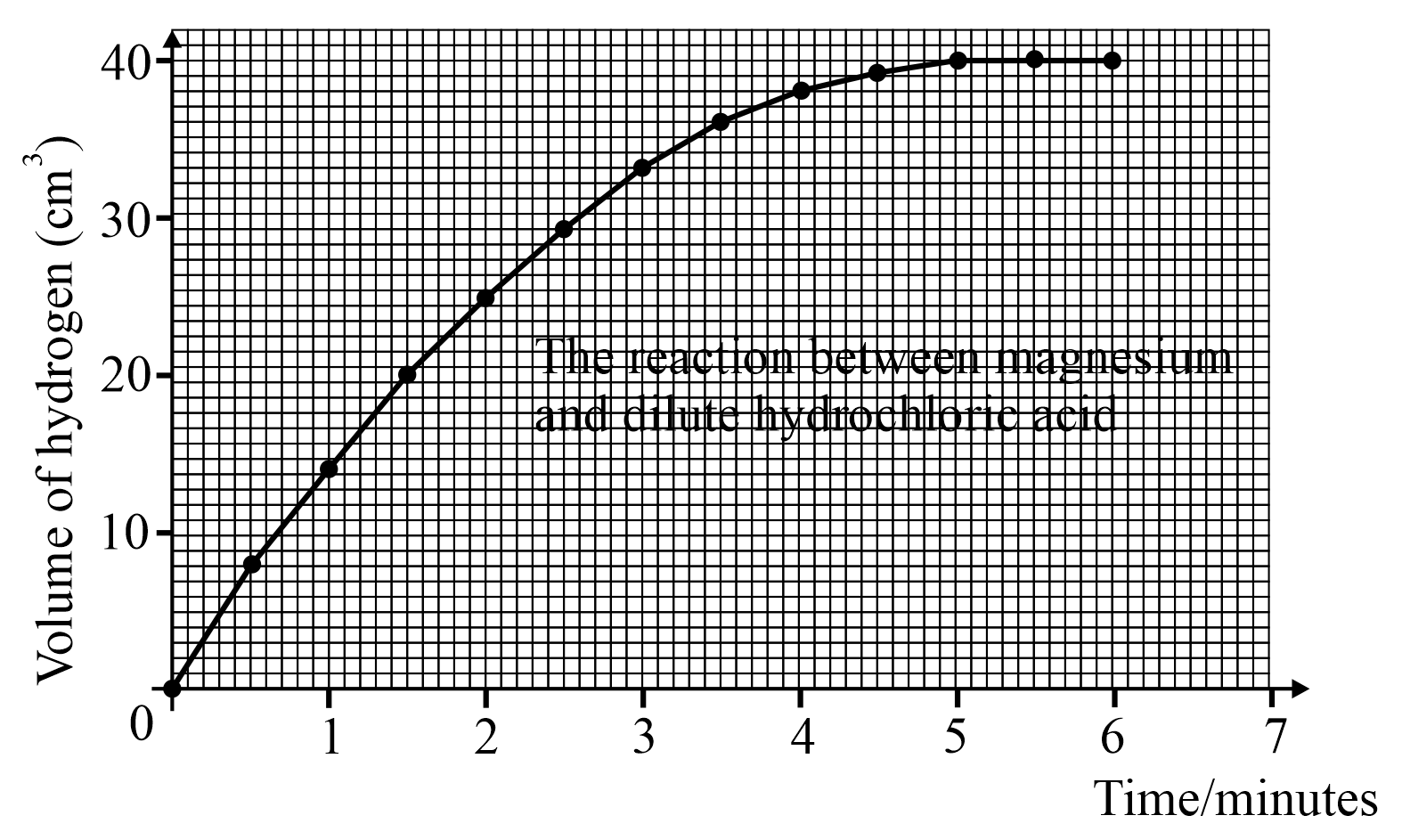
Look at the above graph and answer; how much hydrogen is produced in the first 2.5 minutes?
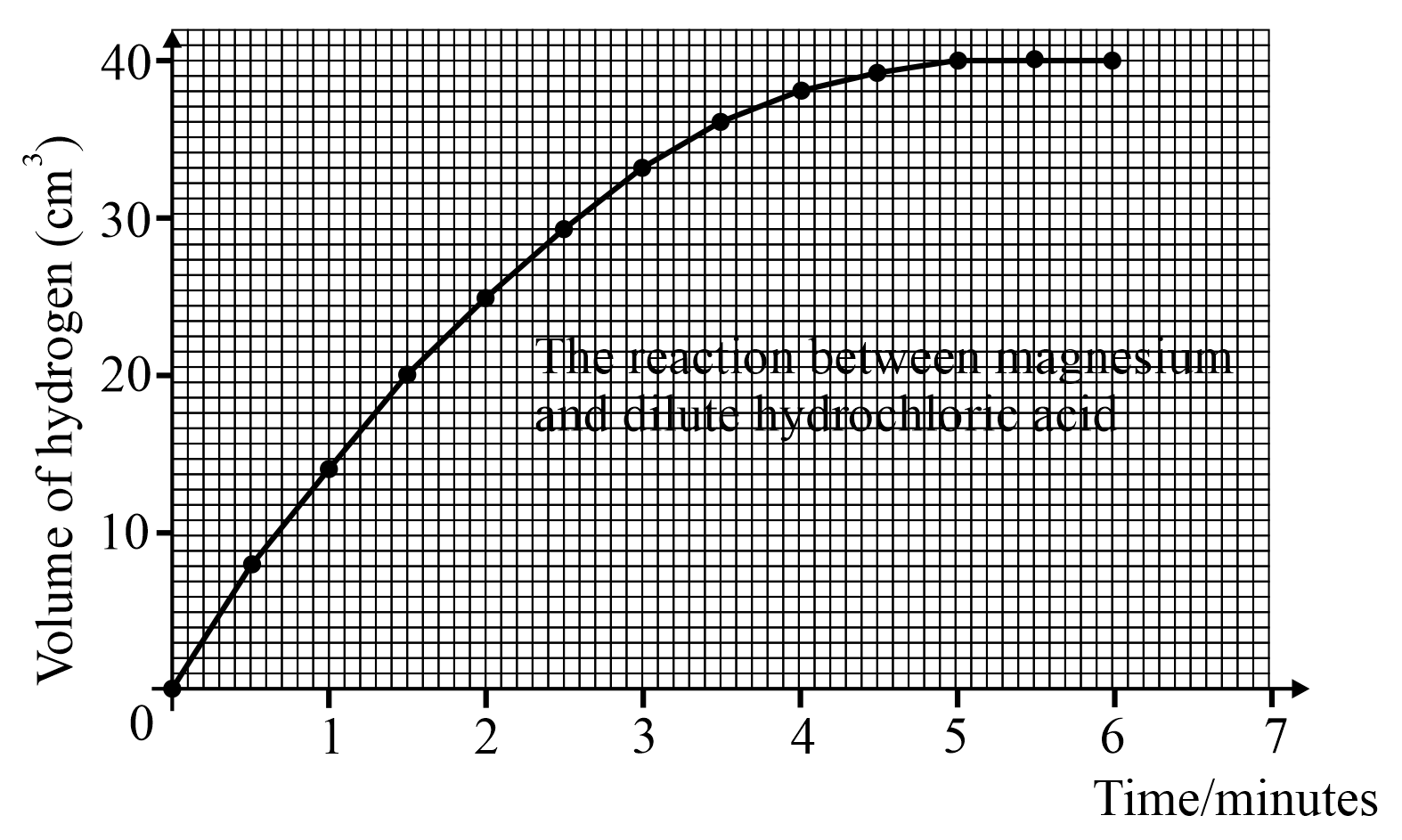
Look at the above graph and answer; how much hydrogen is produced in the first 4.5 minutes?
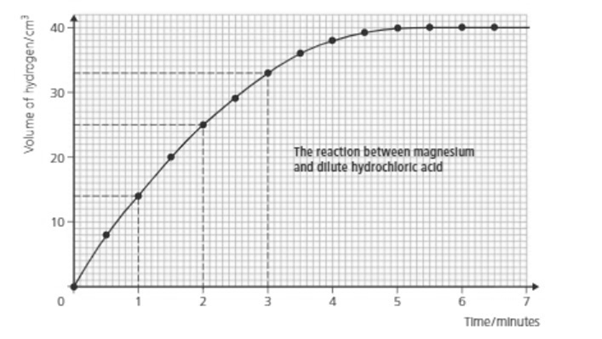
How long did it take to get of hydrogen?
Look at the graph and answer:
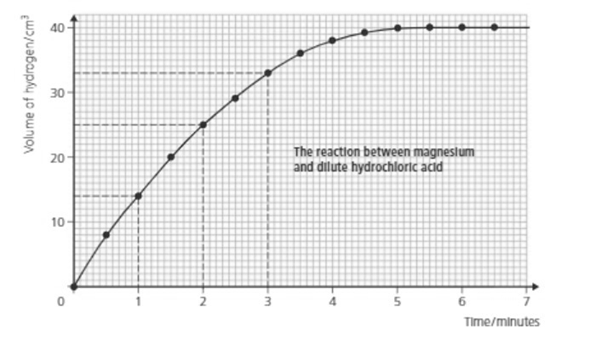
What is the rate of reaction during the fourth minute?
Look at the graph and answer:
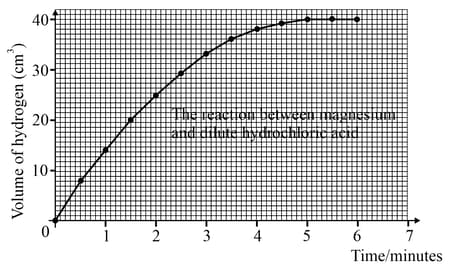
What is the rate of reaction during the sixth minute?
Complete and explain the following:
A reaction goes _____ when the concentration of a ______ is increased. It also goes ______ when the _____ is raised.
