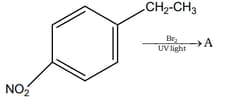
HARD
Earn 100
Convert ethane to ethyl isocyanide.
Important Questions on Hydrocarbons
MEDIUM
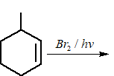
EASY
MEDIUM
Which hydrogen in compound is easily replaceable during bromination reaction in presence of light ?
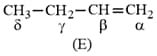
HARD
The total number of monohalogenated organic products in the following (including stereoisomers) reaction is
MEDIUM
EASY
MEDIUM
EASY
Given below are two statements:
Statement-I : -methylbutane on oxidation with gives -methylbutan--ol.
Statement-II : -alkanes can be easily oxidised to corresponding alcohol with .
Choose the correct option :
EASY
MEDIUM
Which of the following is a free radical substitution reaction?
MEDIUM
EASY
MEDIUM
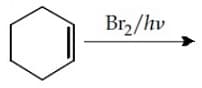
EASY
EASY
Presence of which reagent will affect the reversibility of the following reaction, and change it to a irreversible reaction :
EASY
EASY