HARD
11th ICSE
IMPORTANT
Earn 100
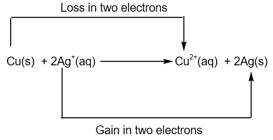
Copper metal, , is oxidised to , while is reduced to silver metal, , then the equilibrium greatly lies in favour of

(a)
(b)
(c)
(d)
20% studentsanswered this correctly

Important Questions on Redox Reactions
HARD
11th ICSE
IMPORTANT
HARD
11th ICSE
IMPORTANT
Which is the best description of behaviour of bromine in the given equation?
HARD
11th ICSE
IMPORTANT
HARD
11th ICSE
IMPORTANT
HARD
11th ICSE
IMPORTANT
For the galvanic cell in which the following reaction takes place, write individual reaction at each electrode.
HARD
11th ICSE
IMPORTANT
Calculate for the cell:
Given, and are and respectively.
HARD
11th ICSE
IMPORTANT
HARD
11th ICSE
IMPORTANT
Balance the following equation
(in acidic medium)
