EASY
JEE Main
IMPORTANT
Earn 100
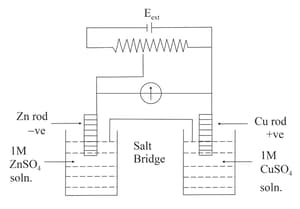
Identify the incorrect statement from the options below for the above cell:
(a)If flow from to
(b)If dissolves at electrode and deposits at electrode
(c)If dissolves at anode and deposits at cathode
(d)If no flow of or current occurs
66.67% studentsanswered this correctly

Important Questions on Electrochemistry
EASY
JEE Main
IMPORTANT
Galvanization is applying a coating of:
MEDIUM
JEE Main
IMPORTANT
Match List - I with List - II :
| List - I (Parameter) |
List - II (Unit) | ||
| (a) | Cell constant | (i) | |
| (b) | Molar conductivity | (ii) | Dimensionless |
| (c) | Conductivity | (iii) | |
| (d) | Degree of dissociation of electrolyte | (iv) |
MEDIUM
JEE Main
IMPORTANT
Consider the following cell reaction
The value of is at If the standard entropy change in is _________ . (Nearest integer)
[Given : Faraday constant ]
MEDIUM
JEE Main
IMPORTANT
An acidic solution of dichromate is electrolysed for minutes using A current. As per the following equation
The amount of obtained was . The efficiency of the process is (Take : , At. mass of chromium )
MEDIUM
JEE Main
IMPORTANT
Let and , be the conductances (in S) measured for saturated aqueous solutions of and respectively, at a temperature T. Which of the following is false?
HARD
JEE Main
IMPORTANT
The photoelectric current from Na (work function, ) is stopped by the output voltage of the cell
the of aq. required to stop the photoelectric current from , all other conditions remaining the same, is (to the nearest integer).
Given
EASY
JEE Main
IMPORTANT
Given
Among the following, the strongest reducing agent is:
EASY
JEE Main
IMPORTANT
For the cell the cell potential . For the cell the cell potential Find value of x (Round off the Nearest Integer).
[ Use ]
