EASY
NEET
IMPORTANT
Earn 100
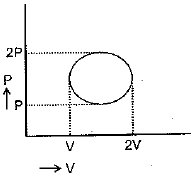
For this cyclic process change in enthalpy of ideal gas is:

(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
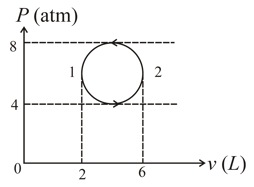
What can be said about work done in going through the cyclic process shown in the adjacent figure:
EASY
NEET
IMPORTANT
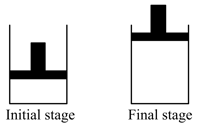
Which is currect one (Dimensions of both the container and piston are identical).
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
