EASY
NEET
IMPORTANT
Earn 100
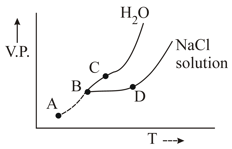
Freezing point of solution is marked as

(a)
(b)
(c)
(d)
60% studentsanswered this correctly

Important Questions on Solutions
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
Correct oder of freezing point of given solution
I. glucose
II. urea
III.
IV.
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
