MEDIUM
Earn 100
How do you identify a strong electrophile?
Important Questions on Organic Chemistry- Some Basic Principles and Techniques
MEDIUM
In which of the following compounds, the bond ionization shall give most stable carbonium ion?
MEDIUM
The correct order of nucleophilicity is
HARD
A solution of in toluene racemises slowly in the presence of a small amount of SbCl5, due to the formation of :
HARD
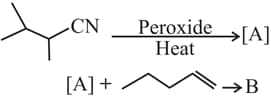
The major products and in the following reactions are
MEDIUM
The increasing order of nucleophilicity of the following nucleophiles is;
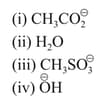
MEDIUM
The compound that is most difficult to protonate is:
EASY
The order of stability of the following carbocations:
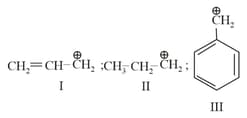
EASY
The lower stability of ethyl anion compared to methyl anion and the higher stability of ethyl radical compared to methyl radical, respectively, are due to
EASY
Which among the following is a set of nucleophiles?
MEDIUM
Given below are two statements :
Statement I : and both can generate nucleophile.
Statement II : and both will generate nitrile nucleophile with all reaction conditions.
Choose the most appropriate option :
EASY
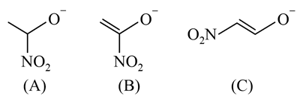
The correct order of stability for the following alkoxides is:
MEDIUM
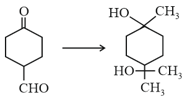
The correct sequence of reagents for the following conversion will be
EASY
The indicated atom is not a nucleophilic site in
MEDIUM
In which of the following pairs is more stable than
EASY
For the following carbocations, the correct order of stability is
I.
II.
III.
MEDIUM
Arrange the following carbanions in order of their decreasing stability
(i)
(ii)
(iii)
MEDIUM
The following compound
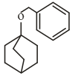
can readily be prepared by Williamson ether synthesis by reaction between
HARD
What will be the correct nucleophilicity order in protic or aprotic solvents?
EASY
The correct statement regarding electrophiles is:
EASY
Which of the following statements is not correct for a nucleophile?

