MEDIUM
Earn 100
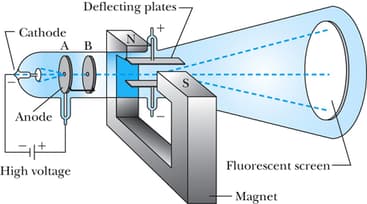
If the given schematic diagram represents Thomson's experiment and the corresponding observation, what would be his atomic model?

Important Questions on Structure of The Atom
EASY
MEDIUM
Match the following :
List -I :
(a) Frequency of distribution of the emitted radiation from a black body
(b) Spin quantum numbers(ms)
(c) Angular Momentum
(d) All orbital have equal energy
List - II :
(i) degeneracy
(ii) temperature dependent
(iii) vector quantity
(iv) mass times velocity times radius
Codes:
MEDIUM
Identify the sets of quantum numbers which are not possible?
EASY
HARD
EASY
-particle scattering experiment was performed by:
HARD
MEDIUM
MEDIUM
Why do electrons not fall into the nucleus?
EASY
In gold foil experiment most of the -particles pass through it, it shows
MEDIUM
What should students need to learn by a specific way of working of scientists?
HARD
EASY
HARD
MEDIUM
MEDIUM
What value can you learn from the ongoing journey of the development of atomic structure from Dalton's model of atom to the present model of atom? Is this value applicable to the improvement of the standard of our life?
EASY
