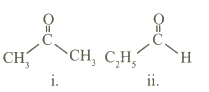
Observe the structural formulae (i) and (ii). Find out their molecular formulae. What is the difference between them? What is the relation between the two compounds represented by these structural formulae?
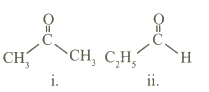

Important Questions on Basic Principles of Organic Chemistry
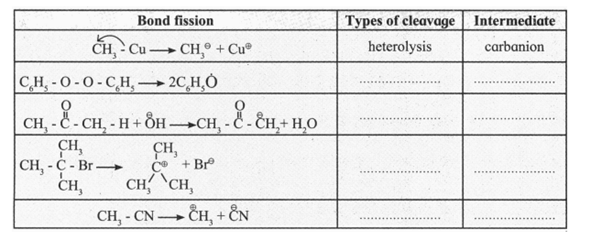
Some bond fissions are described in the following table. For each of them, show the movement of electrons using curved arrow notation. Classify them as homolysis or heterolysis and identify the intermediate species produced as carbocation, carbanion or free radical.
Identify the 'reagent', 'substrate', 'product', and 'by-product' in the following reaction.
How is covalent bond formed between two atoms?
Consider two covalently bonded atoms and where is more electronegative than . Will these atoms share the electron pair equally between them?
Consider two covalently bonded atoms and where is more electronegative than . Represent the polar covalent bond between and using fractional charges and .
