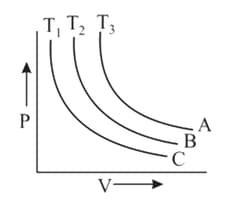
The PV diagram for three different isothermal processes A, B and C are shown above. Chose the correct option.

Important Questions on Thermal Physics
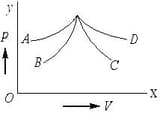
Which of the following is isentropic process
The point marked in the diagram represents the state of a fixed quantity of ideal gas in a container with a moveable piston. The temperature of the gas in the state shown is . Copy the diagram. Indicate on the diagram the point representing separate change.
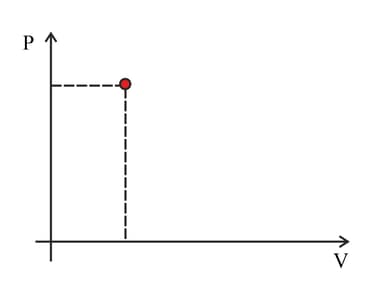
a. The volume doubles at a constant temperature.
The graph for Boyle's law is called
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
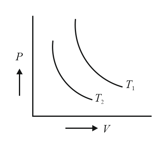
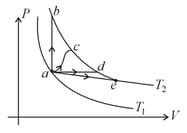
The figure shows two isotherms at temperatures and . A gas is taken from one isotherm to another isotherm through different processes. Then change in internal energy has a relation –
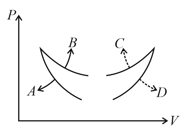
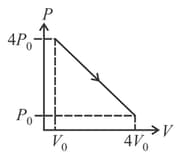
If the indicator diagram for expansion of gas is as shown, the gas
Two isothermals are shown in figure at temperature and . Which of the following relations is correct?