EASY
9th ICSE
IMPORTANT
Earn 100


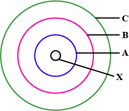
The above sketch is of the
model of an atom. (Bohr's / Rutherford's)



50% studentsanswered this correctly

Important Questions on Atomic Structure and Chemical Bonding
EASY
9th ICSE
IMPORTANT
EASY
9th ICSE
IMPORTANT
EASY
9th ICSE
IMPORTANT
EASY
9th ICSE
IMPORTANT
Complete the table given below by identifying .
| Element | Symbol | No. of Protons | No. of Neutrons | No. of Electrons |
| Sodium | ||||
| Chlorine | ||||
| Uranium | ||||
EASY
9th ICSE
IMPORTANT
EASY
9th ICSE
IMPORTANT
The atomic number and mass number of sodium are respectively. What information is conveyed by this statement?
EASY
9th ICSE
IMPORTANT
Write down the names of the particles represented by the following symbols and explain the meaning of the superscript and subscript numbers mentioned.
EASY
9th ICSE
IMPORTANT
From the symbol state the mass number, atomic number and electronic configuration of magnesium.
