EASY
MHT-CET
IMPORTANT
Earn 100
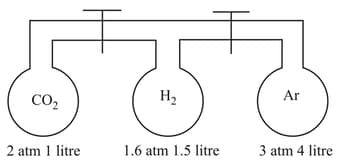
The apparatus shown above consists of three bulbs connected by stop cocks. What is the pressure inside the system, when stop cocks are open? (Assume that temperature remains constant)

(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on States of Matter: Gaseous and Liquid states
EASY
MHT-CET
IMPORTANT
and are two identical vessels. Vessel contains ethane at and , whereas vessel contains of a gas at the same temperature and pressure. What is the molecular weight of ?
EASY
MHT-CET
IMPORTANT
If the pressure and absolute temperature of of are doubled, the volume of would become _____.
MEDIUM
MHT-CET
IMPORTANT
At , the density of a gas, whose molecular weight is is________
EASY
MHT-CET
IMPORTANT
What mass of an oxygen gas will occupy of volume at pressure and temperature?
EASY
MHT-CET
IMPORTANT
Combined gas equation is CORRECTLY represented as ________
EASY
MHT-CET
IMPORTANT
Five grams each of the following gases at pressure are taken. Which of them will have the least volume?
EASY
MHT-CET
IMPORTANT
Which of the following statements is NOT true for an ideal gas?
EASY
MHT-CET
IMPORTANT
Densities of two gases are in the ratio and their temperatures are in the ratio , the ratio of their respective pressures is ( Assume molar masses of both the gases are equal):
