MEDIUM
8th CBSE
IMPORTANT
Earn 100
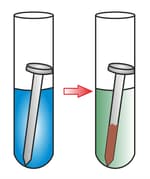
The change in colour of the dipped portion of the iron nail in copper sulphate solution is because of the deposition of _____.

(a)copper
(b)iron
(c)sulphur
(d)aluminium
50% studentsanswered this correctly

Important Questions on Materials: Metals and Non-Metals
MEDIUM
8th CBSE
IMPORTANT
The portion of grey nail which is inside the blue solution of copper sulphate becomes reddish brown.
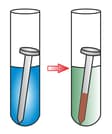
The activity shows ______.
MEDIUM
8th CBSE
IMPORTANT
MEDIUM
8th CBSE
IMPORTANT
MEDIUM
8th CBSE
IMPORTANT
MEDIUM
8th CBSE
IMPORTANT
What happens when the following metals react with dilute hydrochloric acid?
(a) magnesium
MEDIUM
8th CBSE
IMPORTANT
What happens when the following metals react with dilute hydrochloric acid?
(b) Aluminium
MEDIUM
8th CBSE
IMPORTANT
MEDIUM
8th CBSE
IMPORTANT
