The correct relationship between free energy and equilibrium constant K of a reaction is:
The correct relationship between free energy and equilibrium constant K of a reaction is:
Important Questions on Thermodynamics
The standard free energy of the formation of is at . What is the standard free energy of the formation of at ?
at
Hence in is _____________.
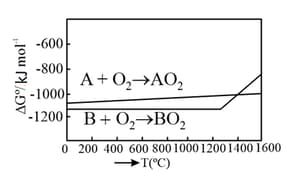
The standard state means that the pressure should be 1 bar, and substance should be pure at a given temperature. The conversion of graphite [ (graphite)] to diamond [ (diamond)] reduces its volume by . If (graphite) is converted to (diamond) isothermally at , the pressure at which (graphite) is in equilibrium with (diamond), is
[Useful information: ]
and are, respectively, and at 298 K. The equilibrium constant for the reaction at 298 k is:
For a dimerization reaction,
at ,then the will be …...
Give an answer to the nearest integer value.
The surface of copper gets tarnished by the formation of copper oxide. gas was passed to prevent the oxide formation during heating of copper at 1250 K. However, the gas contains 1 mole % of water vapour as impurity. The water vapour oxidises copper as per the reaction given below:
Is the minimum partial pressure of (in bar) needed to prevent the oxidation at . The magnitude of value of is ____.
(Given: total pressure = 1 bar, R (universal gas constant) are mutually immiscible.
At
)
Round off the answer up to the nearest integer.
For the reaction,
.
What is the value of free energy change, at for the reaction?

