HARD
Earn 100
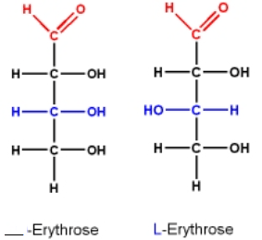
The enantiomer on the left based on configuration is:
-Erythrose.
50% studentsanswered this correctly
Important Questions on Basic principles of Organic Chemistry
EASY
Write R/S configurations of
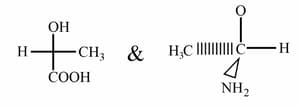
HARD
Newman projections and are shown below:
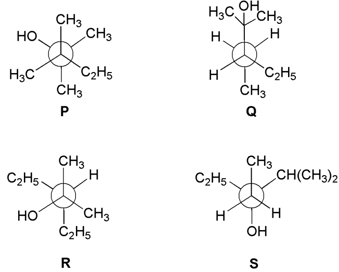
Which one of the following options represent identical molecules?
MEDIUM
Which of the following compounds is (S)--chloro-l-methylcyclohexene?
MEDIUM
In case of R, S configuration the group having highest priority is-
EASY
In assigning R-S configuration which among the following groups has highest priority?
EASY
The configuration of the compound is
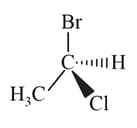
EASY
The configuration of the compound
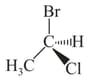
HARD
What is the and configuration for each stereogenic centre in this sugar from top to bottom?
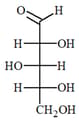
EASY
The absolute configuration of
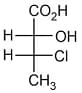 is:
is:EASY
The number of chiral carbons in chloramphenicol is ____________.
MEDIUM
The absolute configurations of the following compounds 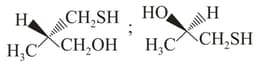 respectively, are
respectively, are
EASY
Two possible stereo-structures of , which are optically active, are called:
MEDIUM
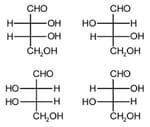
The correct corresponding order of names of four aldoses with configuration given below respectively, is
EASY
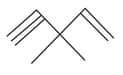
How many pi bonds and sigma bonds are present in following molecule?
EASY
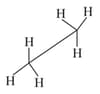
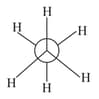
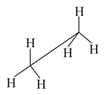
In the following structures, which two forms are the staggered conformation of ethane?
(1) 
(2) 
(3) 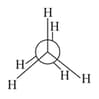
(4) 
HARD
Explain the difference between conformation and configuration necessary condition for geometrical isomerism.
MEDIUM
In the energy diagram, dihedral angle of the conformations of ethane, the staggered forms are found at:
EASY
Draw two Newman projection formulae and two sawhorse formulae for the propane molecule.
MEDIUM
Angle strain in cyclopropane is
HARD
What is conformation? Draw the Newman projection of ethane.

