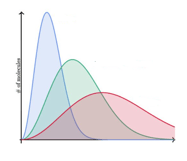
The graph of Maxwell's distribution for three different types of gases (hot gas, cold gas, and gas at room temperature) is shown by three different colors. Determine the correct option.

Red - Gas at room temperatue
Green - Hot gas
Blue - Cold gas
Blue - Cold gas
Green - Gas at room temperature
Red - Hot gas
Green - Hot gas
Blue - Cold gas
Red - Gas at room temperature
Green - Cold gas
Blue - Hot gas
Red - Gas at room temperature
Important Questions on Thermal Physics
a) Temperature of a gas is a measure of average kinetic energy of a molecule
b) Temperature of a gas depends on the nature of the gas
c) Heavier molecule has lower average speed
d) Lighter molecule has lower average speed
The average translational kinetic energy of gas molecules at becomes equal to the of an electron accelerated from rest through a potential difference of
(Consider , (Fill the nearest integer).
Following statements are given
(1) The average kinetic energy of a gas molecule decreases when the temperature is reduced.
(2) The average kinetic energy of a gas molecule increases with increase in pressure at constant temperature.
(3) The average kinetic energy of a gas molecule decreases with increases in volume.
(4) Pressure of a gas increases with increase in temperature at constant pressure.
(5) The volume of gas decreases with increase in temperature.
Choose the correct answer from the options given below :

