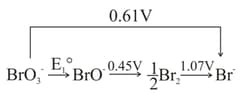The standard e.m.f. of a galvanic cell involving cell reaction with n = 2 is found to be 0.295 V at The equilibrium constant of reaction would be –
The standard e.m.f. of a galvanic cell involving cell reaction with n = 2 is found to be 0.295 V at The equilibrium constant of reaction would be –
Important Questions on Electrochemistry
at is approximately:
The standard emf of the cell and equilibrium constant of the following reaction of
The emf of the following cell is at .
The standard free energy change for the cell reaction is________
is
The standard e.m.f. of the cell, in which the cell reaction is is at and at . The enthalpy change of the reaction at is
For the cell reaction
at The standard Gibbs energy of the cell reaction is:
[Given that Faraday constant ]
Given that:
The standard electrode potential and its temperature coefficient for a cell are and at , respectively. The reaction is . The standard reaction enthalpy at in is
Use and