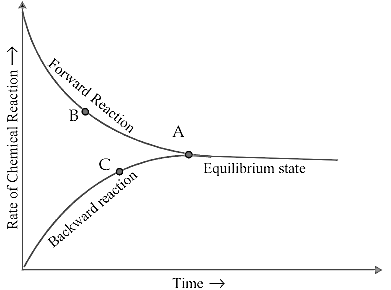
What happens to the rates of forward and backward reaction as time progresses in the given graph of reversible process?


Important Questions on Compounds of Non - Metals
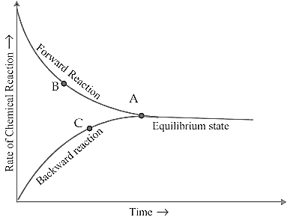
Identify the point at which the rates of both forward and backward reactions become equal in the given graph of a reversible process.
What will happen if the concentration of ammonia is increased in the following reaction?
What will be the effect, if the ammonia produced is removed continuously from the system in the following reaction?
Complete the table by writing the effect of the change in concentration of the system at equilibrium for the following reaction,
| Action | Change of concentration | Change in rate |
| More hydrogen is added | Increases the concentration of the reactant. | Rate of forward reaction increases. |
| More ammonia is added | Increases the concentration of the product. | |
| Ammonia is removed | Decreases the concentration of the product. | |
| More nitrogen is added | Increases the concentration of the reactant. |
In the following equation what is the total number of moles of the reactant molecules?
What are the products in the following reaction?
In the manufacture of ammonia, the reaction in which direction results in the decrease in the number of molecules is?
What happens when the pressure of the system is increased?
