MEDIUM
Earn 100
What is a primary valency ?
Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
EASY
EASY
EASY
EASY
MEDIUM
EASY
MEDIUM
EASY
(i)
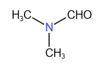 (ii)
(ii) 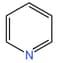
(iii) (iv)
EASY
EASY
MEDIUM
EASY
MEDIUM
EASY
How many (i) hybridised carbon atoms and (ii) bonds are present in the following compound?
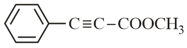
EASY
MEDIUM
The potential energy curve for formation as a function of internuclear distance of the atoms is shown below.
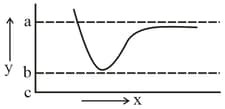
The bond energy of is :
MEDIUM
EASY
MEDIUM

