MEDIUM
Earn 100
What is hybridisation ?
Important Questions on Chemical Bonding
EASY
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
(i)
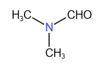 (ii)
(ii) 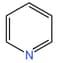
(iii) (iv)
MEDIUM
EASY
Draw the shape of the following molecule:
EASY
EASY
MEDIUM
EASY
EASY
EASY
EASY
EASY
Draw the shape of the following molecule:

