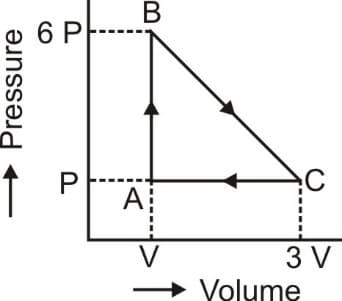
EASY
Earn 100
Which process has maximum work done?
Important Questions on Chemical Thermodynamics
EASY
HARD
MEDIUM
EASY
EASY
EASY
HARD
Match the following
| (A) | Isothermal process | (i) | |
| (B) | Adiabatic process | (ii) | |
| (C) | Isobaric process | (iii) | |
| (D) | Isochoric process | (iv) |
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM
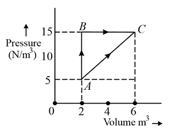
The path along which work done is maximum which
EASY
MEDIUM
MEDIUM
EASY
EASY
EASY