EASY
Earn 100
Why the carbon-carbon bond length is the same in benzene?
Important Questions on Organic Chemistry- Some Basic Principles and Techniques
HARD
The increasing order of basicity of the following compounds is :
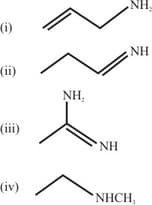
MEDIUM
The hyperconjugative stabilities of tert-butyl cation and 2-butene, respectively, due to
EASY
Among the following oxoacids, the correct decreasing order of acid strength is :
HARD
The increasing order of the of the following compund is:
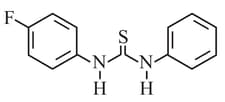
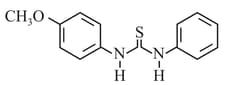
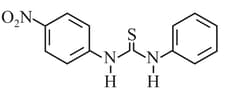
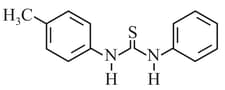
EASY
Consider the following compounds
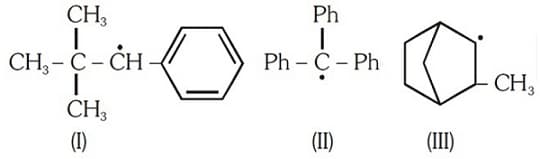
Hyperconjugation occurs in:
HARD
What is the order of basicity among the following compounds?
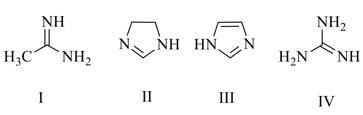
EASY
The correct increasing order of the basic strength for the following compounds is:
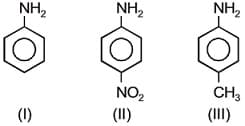
EASY
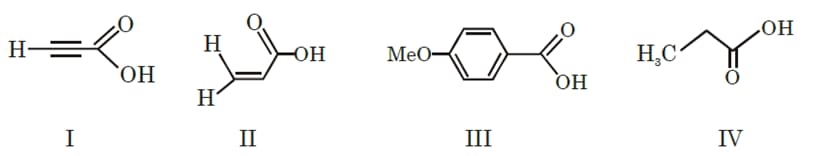
The correct order of acid strength of the following carboxylic acids is -
EASY
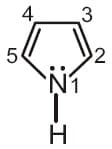
In pyrrole, the electron density is maximum on
MEDIUM
In which of the following molecules, all atoms are coplanar?
HARD
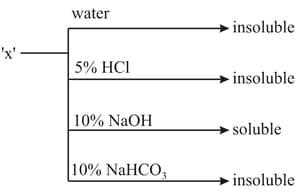
An organic compound showing the following solubility profile is:
MEDIUM
The correct order for acid strength of compounds and is as follows:
MEDIUM
The increasing order of for the following compounds will be :
EASY
The which contribute least to the basicity of the compound is :
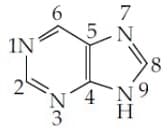
MEDIUM
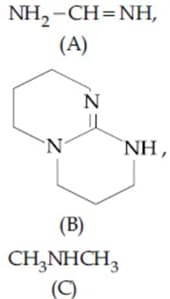
The increasing order of basicity for the following intermediates is (from weak to strong)
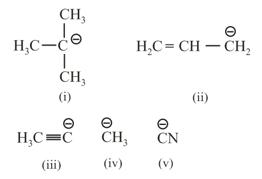
EASY
A tertiary butyl carbocation is more stable than a secondary butyl carbocation because of which of the following?
EASY
Resonance effect is not observed in
EASY
In case of substituted aniline the group which decreases the basic strength is
MEDIUM
The correct order of basicity is
MEDIUM
The correct statement regarding the basicity of aryl amines is:

